Webinar: Mandatory incident reporting within the consumer product safety program
Please note that this event has passed. Please view our upcoming events page or contact events@retailcouncil.org for inquiries about future events.
May 1, 2019
| 1:00pm ET | 12:00pm CT | 10:00am PT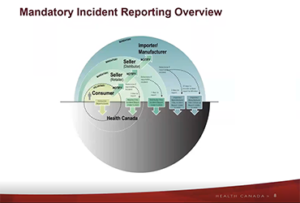 This on-demand webinar outlines the process that health Canada takes once an incident report is received. More specifically, it will answer questions brought forth from RCC and members, discuss product classification (cosmetic vs. consumer product), review incident reporting within legislation (CCPSA and FDA), provide details on the report-intake activities (including prioritization, coding, and other back-end functions), and showcase new surveillance products (e.g. annual report).
This on-demand webinar outlines the process that health Canada takes once an incident report is received. More specifically, it will answer questions brought forth from RCC and members, discuss product classification (cosmetic vs. consumer product), review incident reporting within legislation (CCPSA and FDA), provide details on the report-intake activities (including prioritization, coding, and other back-end functions), and showcase new surveillance products (e.g. annual report). Member only content
This content is locked. Please sign in to access this content.
